Enthalpy changes during phase transformations :
`=>` Phase transformations also involve energy changes.
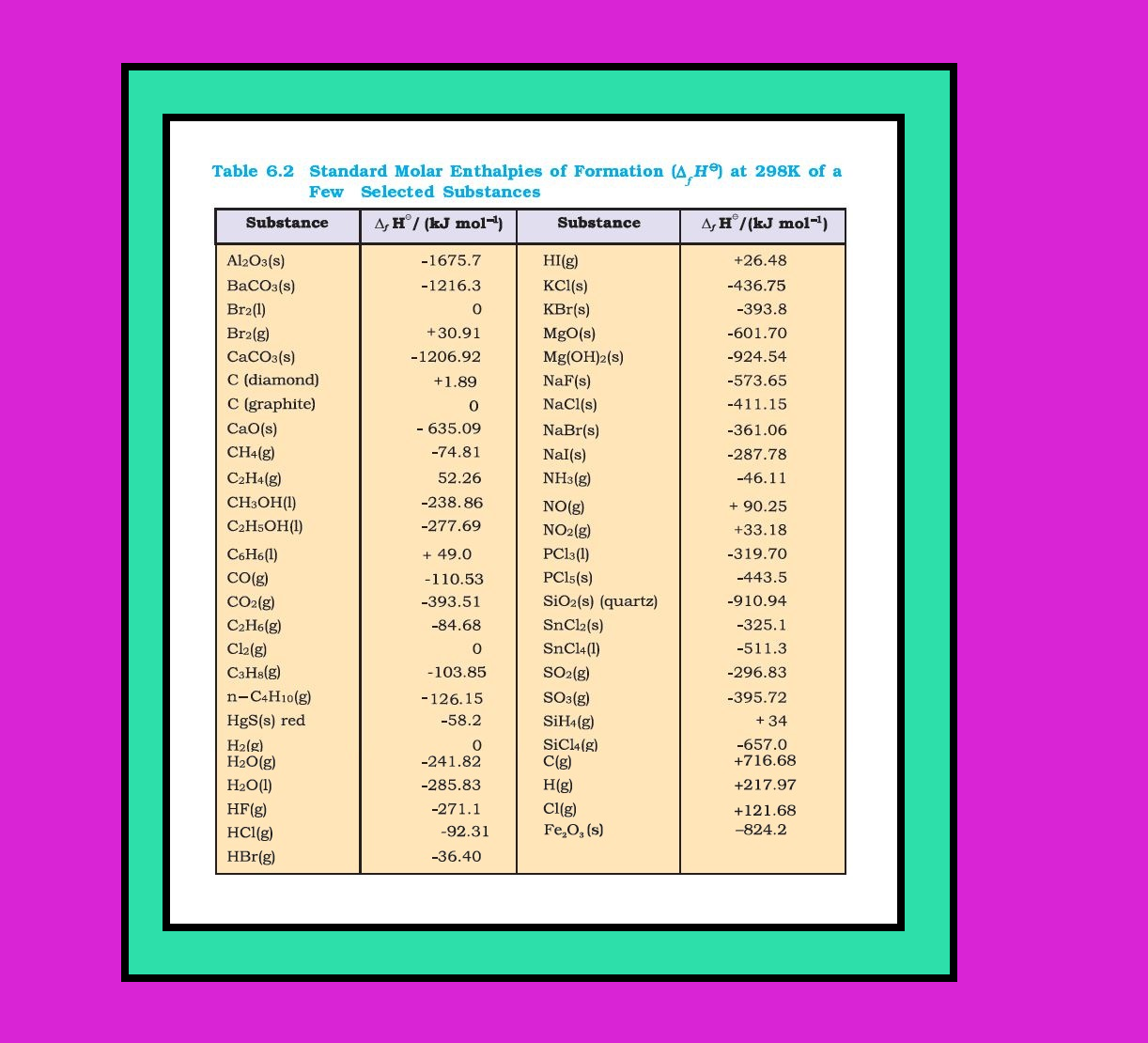
`color{red}("Example ")` Ice requires heat for melting. Normally this melting takes place at constant pressure (atmospheric pressure) and during phase change, temperature remains constant (at `273` `K`).
`color{purple}(H_2O(s) → H_2O(l))`; `color{purple}(Delta_text(fus)H^(⊖) = 6.00kJ mol^(-1))`
● Here `color{purple}(Δ_text(fus) H^(⊖))` is enthalpy of fusion in standard state.
● If water freezes, then process is reversed and equal amount of heat is given off to the surroundings.
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
`color{green}("Standard Enthalpy of Fusion" )` The enthalpy change that accompanies melting of one mole of a solid substance in standard state is called standard enthalpy of fusion or molar enthalpy of fusion, `color{purple}(Δ_text(fus) H^(⊖))`.
● Melting of a solid is endothermic, so all enthalpies of fusion are positive.
`=>` Water requires heat for evaporation. At constant temperature of its boiling point `T_b` and at constant pressure :
`color{purple}(H_2O (l) → H_2O(g) ; Delta_text(vap) H^(⊖) = +40.79 kJ mol^(-1))`
`color{purple}(Δ_text(vap)H^(⊖))` is the standard enthalpy of vaporization.
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
`color{green}("Standard Enthalpy of Vaporization ")` Amount of heat required to vaporize one mole of a liquid at constant temperature and under standard pressure (1bar) is called its standard enthalpy of vaporization or molar enthalpy of vaporization, `Δ_text(vap)H^(⊖)`
`=>` Sublimation is direct conversion of a solid into its vapour.
● Solid `color{purple}(CO_2)` or ‘dry ice’ sublimes at `195` `K` with `color{purple}(Δ_text(sub)H^⊖=25.2 kJ mol^(–1))`; naphthalene sublimes slowly and for this `color{purple}(Delta_text(sub) H^⊖ = 73.0kJmol^(-1))`
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
`color{green}("Standard Enthalpy of Sublimation ")` Standard enthalpy of sublimation, `color{purple}(Δ_text(sub)H^⊖)` is the change in enthalpy when one mole of a solid substance sublimes at a constant temperature and under standard pressure (`1` bar).
`color{red}("Note ")` The magnitude of the enthalpy change depends on the strength of the intermolecular interactions in the substance undergoing the phase transfomations.
`color{red}("Example ")` The strong hydrogen bonds between water molecules hold them tightly in liquid phase. For an organic liquid, such as acetone, the intermolecular dipole-dipole interactions are significantly weaker. Thus, it requires less heat to vaporise `1` mol of acetone than it does to vaporize `1` mol of water.
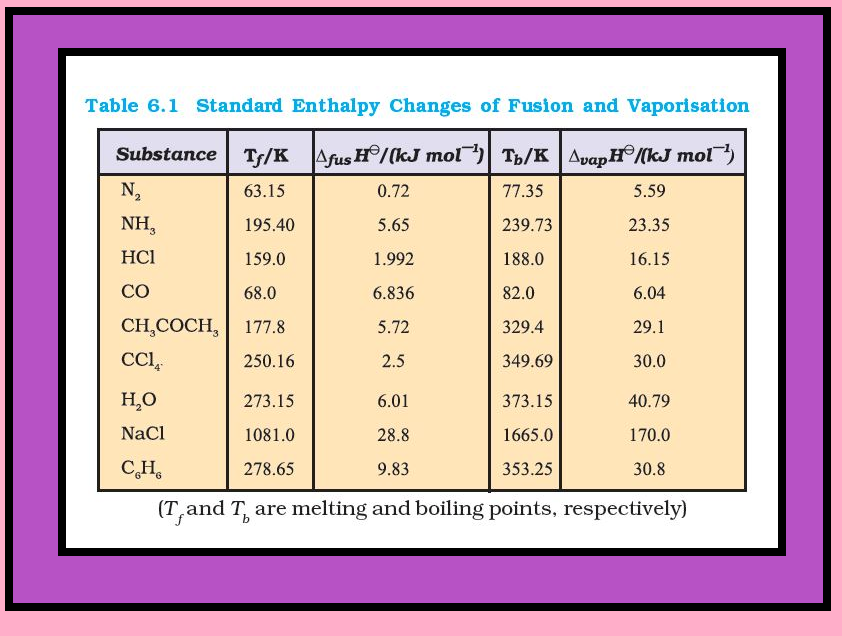
`=>` Table 6.1 gives values of standard enthalpy changes of fusion and vaporisation for some substances.
`color{red}("Example ")` Ice requires heat for melting. Normally this melting takes place at constant pressure (atmospheric pressure) and during phase change, temperature remains constant (at `273` `K`).
`color{purple}(H_2O(s) → H_2O(l))`; `color{purple}(Delta_text(fus)H^(⊖) = 6.00kJ mol^(-1))`
● Here `color{purple}(Δ_text(fus) H^(⊖))` is enthalpy of fusion in standard state.
● If water freezes, then process is reversed and equal amount of heat is given off to the surroundings.
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
`color{green}("Standard Enthalpy of Fusion" )` The enthalpy change that accompanies melting of one mole of a solid substance in standard state is called standard enthalpy of fusion or molar enthalpy of fusion, `color{purple}(Δ_text(fus) H^(⊖))`.
● Melting of a solid is endothermic, so all enthalpies of fusion are positive.
`=>` Water requires heat for evaporation. At constant temperature of its boiling point `T_b` and at constant pressure :
`color{purple}(H_2O (l) → H_2O(g) ; Delta_text(vap) H^(⊖) = +40.79 kJ mol^(-1))`
`color{purple}(Δ_text(vap)H^(⊖))` is the standard enthalpy of vaporization.
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
`color{green}("Standard Enthalpy of Vaporization ")` Amount of heat required to vaporize one mole of a liquid at constant temperature and under standard pressure (1bar) is called its standard enthalpy of vaporization or molar enthalpy of vaporization, `Δ_text(vap)H^(⊖)`
`=>` Sublimation is direct conversion of a solid into its vapour.
● Solid `color{purple}(CO_2)` or ‘dry ice’ sublimes at `195` `K` with `color{purple}(Δ_text(sub)H^⊖=25.2 kJ mol^(–1))`; naphthalene sublimes slowly and for this `color{purple}(Delta_text(sub) H^⊖ = 73.0kJmol^(-1))`
`color{purple}(✓✓)color{purple} " DEFINITION ALERT"`
`color{green}("Standard Enthalpy of Sublimation ")` Standard enthalpy of sublimation, `color{purple}(Δ_text(sub)H^⊖)` is the change in enthalpy when one mole of a solid substance sublimes at a constant temperature and under standard pressure (`1` bar).
`color{red}("Note ")` The magnitude of the enthalpy change depends on the strength of the intermolecular interactions in the substance undergoing the phase transfomations.
`color{red}("Example ")` The strong hydrogen bonds between water molecules hold them tightly in liquid phase. For an organic liquid, such as acetone, the intermolecular dipole-dipole interactions are significantly weaker. Thus, it requires less heat to vaporise `1` mol of acetone than it does to vaporize `1` mol of water.
`=>` Table 6.1 gives values of standard enthalpy changes of fusion and vaporisation for some substances.